
Thermal conductivity
Data table for metals, liquid, gases
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. Thermal conductivity of metals in between 20°c and 800°c
The thermal conductivity of a material, expressed in kcal/h.m.c or W/m.K is measuring how easily a material is able to transmit heat by conduction.
- Warning 1 : values were not verified individually
- Warning 2 : The data below are thus only for a 1st reference, quick calculation, but no detail design, no guarantee are given upon them.
1. Thermal conductivity of metals in between 20°c and 800°c
In between 20 and 500c :
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
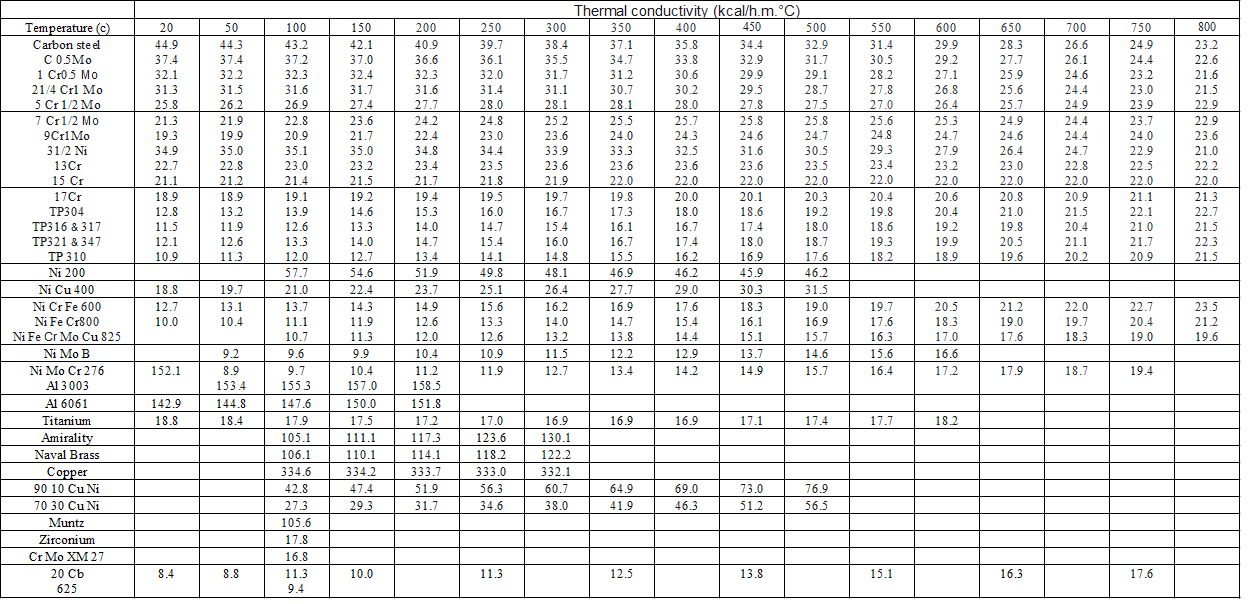
| Thermal conductivity (kcal/h.m.°c) | |||||||||||
| Temperature (c) | 20 | 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 |
| Carbon
steel C 0.5Mo 1 Cr0.5 Mo 21/4 Cr1 Mo 5 Cr 1/2 Mo |
44.9 37.4 32.1 31.3 25.8 |
44.3 37.4 32.2 31.5 26.2 |
43.2 37.2 32.3 31.6 26.9 |
42.1 37.0 32.4 31.7 27.4 |
40.9 36.6 32.3 31.6 27.7 |
39.7 36.1 32.0 31.4 28.0 |
38.4 35.5 31.7 31.1 28.1 |
37.1 34.7 31.2 30.7 28.1 |
35.8 33.8 30.6 30.2 28.0 |
34.4 32.9 29.9 29.5 27.8 |
32.9 31.7 29.1 28.7 27.5 |
| 7
Cr 1/2 Mo 9Cr1Mo 31/2 Ni 13Cr 15 Cr |
21.3 19.3 34.9 22.7 21.1 |
21.9 19.9 35.0 22.8 21.2 |
22.8 20.9 35.1 23.0 21.4 |
23.6 21.7 35.0 23.2 21.5 |
24.2 22.4 34.8 23.4 21.7 |
24.8 23.0 34.4 23.5 21.8 |
25.2 23.6 33.9 23.6 21.9 |
25.5 24.0 33.3 23.6 22.0 |
25.7 24.3 32.5 23.6 22.0 |
25.8 24.6 31.6 23.6 22.0 |
25.8 24.7 30.5 23.5 22.0 |
| 17Cr TP304 TP316 & 317 TP321 & 347 TP 310 |
18.9 12.8 11.5 12.1 10.9 |
18.9 13.2 11.9 12.6 11.3 |
19.1 13.9 12.6 13.3 12.0 |
19.2 14.6 13.3 14.0 12.7 |
19.4 15.3 14.0 14.7 13.4 |
19.5 16.0 14.7 15.4 14.1 |
19.7 16.7 15.4 16.0 14.8 |
19.8 17.3 16.1 16.7 15.5 |
20.0 18.0 16.7 17.4 16.2 |
20.1 18.6 17.4 18.0 16.9 |
20.3 19.2 18.0 18.7 17.6 |
| Ni 200 | 57.7 | 54.6 | 51.9 | 49.8 | 48.1 | 46.9 | 46.2 | 45.9 | 46.2 | ||
| Ni Cu 400 | 18.8 | 19.7 | 21.0 | 22.4 | 23.7 | 25.1 | 26.4 | 27.7 | 29.0 | 30.3 | 31.5 |
| Ni
Cr Fe 600 Ni Fe Cr800 Ni Fe Cr Mo Cu 825 |
12.7 10.0 |
13.1 10.4 |
13.7 11.1 10.7 |
14.3 11.9 11.3 |
14.9 12.6 12.0 |
15.6 13.3 12.6 |
16.2 14.0 13.2 |
16.9 14.7 13.8 |
17.6 15.4 14.4 |
18.3 16.1 15.1 |
19.0 16.9 15.7 |
| Ni Mo B | 9.2 | 9.6 | 9.9 | 10.4 | 10.9 | 11.5 | 12.2 | 12.9 | 13.7 | 14.6 | |
| Ni
Mo Cr 276 Al 3003 |
152.1 | 8.9 153.4 |
9.7 155.3 |
10.4 157.0 |
11.2 158.5 |
11.9 | 12.7 | 13.4 | 14.2 | 14.9 | 15.7 |
| Al 6061 | 142.9 | 144.8 | 147.6 | 150.0 | 151.8 | ||||||
| Titanium | 18.8 | 18.4 | 17.9 | 17.5 | 17.2 | 17.0 | 16.9 | 16.9 | 16.9 | 17.1 | 17.4 |
| Amirality | 105.1 | 111.1 | 117.3 | 123.6 | 130.1 | ||||||
| Naval Brass | 106.1 | 110.1 | 114.1 | 118.2 | 122.2 | ||||||
| Copper | 334.6 | 334.2 | 333.7 | 333.0 | 332.1 | ||||||
| 90 10 Cu Ni | 42.8 | 47.4 | 51.9 | 56.3 | 60.7 | 64.9 | 69.0 | 73.0 | 76.9 | ||
| 70 30 Cu Ni | 27.3 | 29.3 | 31.7 | 34.6 | 38.0 | 41.9 | 46.3 | 51.2 | 56.5 | ||
| Muntz | 105.6 | ||||||||||
| Zirconium | 17.8 | ||||||||||
| Cr Mo XM 27 | 16.8 | ||||||||||
| 20
Cb 625 |
8.4 | 8.8 | 11.3 9.4 |
10.0 | 11.3 | 12.5 | 13.8 | ||||
Full range 20-800c :
You can download the image or download
the Excel file here.
2. Thermal conductivity of liquids
The thermal conductivity of common liquids is given in the table below. The temperatures given correspond to the range of validity of the values, a linear variation in between temperatures given can be assumed.
| Material (liquid) | T (⁰c) | λ (kcal/h.m.⁰c) | Material (liquid) | T (⁰c) | λ (kcal/h.m.⁰c) |
| Acetic Acid | 20 | 0.137 | Formaldehyde | -80 | 0.275 |
| 150 | 0.116 | -20 | 0.196 | ||
| Acetone | -20 | 0.138 | 20 | 0.172 | |
| 80 | 0.113 | Glycerine | 20 | 0.239 | |
| Acetylene | -140 | 0.204 | 200 | 0.269 | |
| -80 | 0.132 | Heptane (n-) | 10 | 0.11 | |
| 0 | 0.085 | 150 | 0.074 | ||
| Acrylic acid | 0 | 0.0214 | Hexane (n-) | 10 | 0.107 |
| 40 | 0.184 | 150 | 0.068 | ||
| 160 | 0.128 | Heptyl alcohol | 20 | 0.114 | |
| Allyl alcohol | 20 | 0.141 | 140 | 0.106 | |
| 100 | 0.137 | Hexyl alcohol | 20 | 0.114 | |
| Amyl alcohol | 20 | 0.132 | 120 | 0.11 | |
| 100 | 0.126 | Methylthyl ketone (MEK) | -20 | 0.132 | |
| Aniline | 20 | 0.198 | 120 | 0.1 | |
| 150 | 0.132 | Nonane (n-) | 10 | 0.114 | |
| Benzene | 20 | 0.126 | 150 | 0.083 | |
| 160 | 0.088 | Octane | 10 | 0.113 | |
| Bromobenzene | 0 | 0.097 | 150 | 0.08 | |
| 200 | 0.088 | Para xylene | 20 | 0.113 | |
| Butyl acetate (n-) | 0 | 0.122 | 80 | 0.097 | |
| 160 | 0.083 | 200 | 0.07 | ||
| Butyl alcohol (iso-) | -40 | 0.149 | Pentane | 10 | 0.103 |
| 10 | 0.129 | 120 | 0.071 | ||
| 70 | 0.114 | Propyl alcohol (n-) | -40 | 0.158 | |
| 150 | 0.111 | 150 | 0.107 | ||
| Butyl alcohol (n-) | -40 | 0.155 | Propyl alcohol (iso-) | -40 | 0.137 |
| 150 | 0.095 | 60 | 0.112 | ||
| Carbon disulfide | -80 | 0.125 | 150 | 0.107 | |
| 20 | 0.107 | Toluene | 0 | 0.123 | |
| Carbon tetrachloride | -80 | 0.106 | 200 | 0.074 | |
| 100 | 0.077 | Trichlorethylene | -40 | 0.125 | |
| Chlorobenzene | 0 | 0.112 | 30 | 0.097 | |
| 200 | 0.101 | 150 | 0.068 | ||
| Chloroform | -75 | 0.123 | Vinyl acetate | 0 | 0.131 |
| 100 | 0.083 | 110 | 0.097 | ||
| Cumene | 0 | 0.112 | Water | 0 | 0.51 |
| 200 | 0.074 | 40 | 0.54 | ||
| Cyclohexane | 5 | 0.132 | 95 | 0.57 | |
| 40 | 0.12 | 150 | 0.587 | ||
| 120 | 0.089 | 215 | 0.559 | ||
| Dichlorodifluoromethane | -60 | 0.098 | 325 | 0.409 | |
| 10 | 0.094 | Xylene (ortho) | 0 | 0.129 | |
| 60 | 0.086 | 80 | 0.101 | ||
| Ethyl acetate | 0 | 0.131 | 200 | 0.071 | |
| 110 | 0.097 | Xylene (meta) | 0 | 0.119 | |
| Ethyl alcohol | -40 | 0.164 | 80 | 0.092 | |
| 150 | 0.119 | 200 | 0.065 | ||
| Ethyl benzene | 0 | 0.119 | |||
| 200 | 0.0667 |
3. Thermal conductivity of gases
| Thermal conductivity (kcal/h.m.ºc) | ||||||||
| Temperature (ºc) | -200 | -100 | 0 | 50 | 100 | 200 | 300 | 400 |
| Acetone | 0.0085 | 0.0113 | 0.0147 | 0.0233 | ||||
| Acetylene | 0.0083 | 0.0161 | 0.0208 | 0.0256 | ||||
| Air | 0.0059 | 0.0135 | 0.0208 | 0.0274 | 0.0333 | 0.0387 | ||
| Ammonia | 0.0144 (-50c) | 0.0187 | 0.0286 | 0.0416 | 0.0572 | 0.0757 | ||
| Argon | 0.0094 | 0.0141 | 0.0183 | 0.022 | 0.0254 | |||
| Benzen | 0.0077 | 0.0112 | 0.0153 | 0.0247 | ||||
| Butane (n-) | 0.0116 | 0.0201 | ||||||
| Butane (iso-) | 0.0119 | 0.0207 | ||||||
| Carbon dioxide | 0.0095 (-50c) | 0.0125 | 0.019 | 0.0263 | 0.0341 | |||
| Carbon disulfide | 0.0059 | |||||||
| Carbon monoxide | 0.0055 | 0.0131 | 0.0199 | 0.0262 | ||||
| Carbon tetrachloride | 0.0062 | 0.0077 | 0.0101 | |||||
| Chlorine | 0.0064 | |||||||
| Chloroform | 0.0057 | 0.0086 | 0.012 | |||||
| Cyclohexane | 0.014 | |||||||
| Dichlorodifluoromethane | 0.0071 | 0.0095 | 0.0119 | 0.0171 | ||||
| Ethane | 0.0082 | 0.0158 | 0.026 | |||||
| Ethyl acetate | 0.011 | 0.0143 | 0.0223 | |||||
| Ethyl alcohol | 0.012 | 0.0184 | ||||||
| Ethyl chloride | 0.0082 | 0.0141 | 0.0216 | |||||
| Ethyl ether | 0.0114 | 0.015 | 0.0195 | 0.0297 | ||||
| Ethylene | 0.0076 | 0.015 | 0.0195 | 0.0239 | ||||
| Helium | 0.0503 | 0.091 | 0.1216 | 0.1469 | ||||
| Heptane (n-) | 0.0153 | 0.0167 | ||||||
| Hexane (n-) | 0.0107 | 0.0119 (20ºc) | ||||||
| Hexene | 0.0091 | 0.0162 | ||||||
| Hydrogen | 0.0436 | 0.097 | 0.1436 | 0.1844 | 0.2207 | 0.2535 | ||
| Hydrogen sulfide | 0.0113 | |||||||
| Mercury | 0.0293 | |||||||
| Methane | 0.0067 | 0.0162 | 0.0262 | 0.0379 | 0.0532 | 0.0729 | ||
| Methyl acetate | 0.0088 | 0.0101 | ||||||
| Methyl alcohol | 0.0123 | 0.019 | ||||||
| Methyl chloride | 0.0079 | 0.011 | 0.014 | 0.0208 | ||||
| Methylene chloride | 0.0058 | 0.0074 | 0.0094 | 0.0135 | ||||
| Neon | 0.0039 | |||||||
| Nitric oxide | 0.0132 | 0.0205 | 0.0239 | |||||
| Nitrogen | 0.0059 | 0.0135 | 0.0207 | 0.0269 | 0.0327 | 0.0379 | 0.0427 | |
| Nitrous oxide | 0.007 | 0.0131 | 0.0205 | |||||
| Oxygen | 0.0057 | 0.0135 | 0.0211 | 0.0247 | 0.028 | |||
| Pentane (n-) | 0.011 | 0.0123 (20ºc) | ||||||
| Pentane (iso-) | 0.0107 | 0.0189 | ||||||
| Propane | 0.0129 | 0.0225 | ||||||
| Sulfur dioxide | 0.0074 | 0.0103 | ||||||
| Water vapor | 0.0202 | 0.0271 | 0.0342 | 0.0415 | ||||