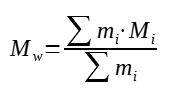Polymers : molar mass, number average, mass average
calculation, polydispersity
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. Number average molar mass
2. Mass average molar mass
3. Polydispersity index
4. Analytical methods to determine a polymer molar mass
5. Impact of molecular mass of polymers
Polymers are, as the name indicates, made of several (poly) monomers linked together which can lead to very long chains and very high molar masses. As each polymer in a reactive media has a different length and thus molar mass, how to calculate the molar mass of a particular polymer ?
There are actually several ways to calculate a molar mass depending on what we give priority to.
1. Number average molar mass
The first method is to count the number of polymers having a given mass, then average over the total count of the polymer chains, this gives the number average molar mass Mn
Polymer number average molar mass calculation formula :
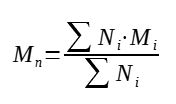
With :
Mn = number average molar mass (g/mol)
Ni = number of polymers having the mass Mi (-)
Mi = molar mass i (g/mol)
The number average molar mass is heavily influenced by small molar masses that are likely to be in larger numbers.
2. Mass average molar mass
Instead of averaging on the number of chains of mass Mi, the average can be on the mass of the polymer chains of mass Mi
Polymer mass average molar mass calculation formula :
With : Mw = mass average molar mass (g/mol)
mi = mass of the polymer chain of molar mass Mi (-)
Mi = molar mass i (g/mol)
As the mass of long chains is much higher than the one of long chains, the mass average molar mass will give an advantage to long chains, thus :
Mw > Mn
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
This can be illustrated in the following typical molar mass distribution graph :

3. Polydispersity index
The polydispersity index is the ratio in between the mass average molar mass and the number average molar mass. It will measure the spread in between both averages and will thus give an indication on how wide is the distribution of molar mass for a given polymer.
Polymer polydispersity index formula :
I = Mw / Mn
As we know that Mw > Mn, then I > 1
Typical values of the polydispersity index are :
| Polydispersity index |
Type of polymerization |
| I > 1.5 | Radical polymerization |
| I <= 2 | Step polymerization |
4. Analytical methods to determine a polymer molar mass
In an industrial setting, engineers routinely employ several sophisticated techniques to measure or calculate the molar mass of polymers, each offering unique insights into the polymer's characteristics. Gel Permeation Chromatography (GPC), also known as Size Exclusion Chromatography (SEC), is one of the most widely used methods. GPC separates polymer molecules based on their size, allowing for the determination of both the number average and weight average molar masses. This technique is particularly valuable as it provides a complete distribution of molar masses within a sample, which is crucial for understanding the polydispersity and overall behavior of the polymer. Another common method is viscometry, which measures the viscosity of a polymer solution to estimate its molar mass. Although less detailed than GPC, viscometry is a cost-effective and quick method for obtaining useful data on polymer size.
Engineers leverage these molar mass calculations to predict how polymers will behave under various conditions, such as changes in temperature or mechanical stress. For instance, knowing the molar mass distribution helps in anticipating the mechanical properties of a polymer, such as tensile strength and elasticity. Higher molar mass polymers generally exhibit greater strength and toughness, making them suitable for demanding applications. Conversely, lower molar mass polymers might be used where flexibility and easier processing are required. Additionally, understanding the molar mass can aid in predicting thermal properties, such as the glass transition temperature, which is critical for applications involving temperature variations.
5. Impact of molecular mass of polymers
The molar mass of a polymer significantly influences its physical properties, which in turn dictate its suitability for various applications. For instance, tensile strength and elasticity are directly affected by the molar mass. Generally, polymers with higher molar masses exhibit greater tensile strength due to increased chain entanglements, which enhance the material's resistance to deformation. This makes high molar mass polymers ideal for applications requiring durability and strength, such as structural components in automotive or aerospace industries. Conversely, lower molar mass polymers tend to be less strong but more flexible and easier to process, making them suitable for applications like packaging films or adhesives.
Elasticity, or the ability of a polymer to stretch and return to its original shape, is also influenced by molar mass. Higher molar mass polymers typically show improved elasticity due to the increased length and entanglement of polymer chains, which can more effectively absorb and distribute applied stresses. This property is crucial in applications such as elastic fibers and flexible seals.
Viscosity, another critical property, is highly dependent on molar
mass, particularly in the molten state. Higher molar mass polymers
generally have higher melt viscosities, which can complicate
processing techniques like extrusion or injection molding. These
processes require the polymer to flow easily into molds or through
dies, and high viscosity can lead to increased energy consumption
and processing difficulties. However, a higher melt viscosity can
also be beneficial in maintaining the structural integrity of the
final product during cooling and solidification.
Variations in molar mass distribution can further influence
processing conditions. A narrow molar mass distribution typically
results in more uniform processing behavior, as the polymer chains
are more consistent in length. This can lead to more predictable
flow characteristics and mechanical properties in the final product.
In contrast, a broad molar mass distribution can offer a balance
between processability and mechanical performance, as the presence
of shorter chains can improve flow while longer chains contribute to
strength and toughness.
In extrusion and injection molding, understanding and controlling the molar mass distribution is essential for optimizing processing parameters such as temperature, pressure, and shear rate. For example, polymers with a broad distribution might require careful adjustment of processing temperatures to ensure that both high and low molar mass components are adequately melted and mixed. This balance is crucial for achieving the desired properties in the final product while maintaining efficient and cost-effective production processes. By carefully tailoring the molar mass and its distribution, engineers can optimize polymer materials for specific applications, enhancing performance and processing efficiency.
Typical molar mass ranges for different polymer applications and materials:
| Application | Typical Molar Mass Range (g/mol) |
|---|---|
| Plastic bags and bottles | 100,000 – 250,000 |
| Tough electronic components | 50,000 – 300,000 |
| Structural components for transit applications | 50,000 – 300,000 |
| Automotive sector (interior textiles) | 10,000 – 50,000 |
| Biomedical materials | 3,000,000 – 6,000,000 |
| Flexible seals and elastic fibers | Higher molar mass (varies, generally high) |
| Non-woven fabrics | 10,000 – 50,000 |
| Severe operating conditions (e.g., UHMWPE) | 2,000,000 – 6,000,000 |
| High-strength engineering polymer | 100,000 – 250,000 |
| Packaging materials | 10,000 – 50,000 |
| Polymer Material | Typical Molar Mass Range (g/mol) |
|---|---|
| High-Density Polyethylene (HDPE) | 100,000 – 250,000 |
| Polycarbonate (PC) | 50,000 – 300,000 |
| Polyamide (Nylon 6) | 10,000 – 50,000 |
| Polyurethane (PU) | 50,000 – 300,000 |
| Ultra-High Molecular Weight Polyethylene (UHMWPE) | 2,000,000 – 6,000,000 |
| Polystyrene and Polycarbonate (for solubility in organic media) | Varies, typically high |
| Polyetherols and Polyesterols | Higher molar mass |
| Polybutadiene (for automotive applications) | Varies based on application |
FAQ: Polymers - Molar Mass, Polydispersity, and Applications
1. What is the number average molar mass (\( M_n \)) of a polymer?
\( M_n \) is calculated by averaging the molar masses of all polymer chains based on their count. It is influenced heavily by smaller molar masses, which are typically more numerous. The formula is: \[ M_n = \frac{\sum N_i \cdot M_i}{\sum N_i} \] Where \( N_i \) is the number of polymers with molar mass \( M_i \).
2. What is the mass average molar mass (\( M_w \)) of a polymer?
\( M_w \) is calculated by averaging the molar masses of all polymer chains based on their mass. It gives more weight to longer chains. The formula is: \[ M_w = \frac{\sum m_i \cdot M_i}{\sum m_i} \] Where \( m_i \) is the mass of polymers with molar mass \( M_i \). Since \( M_w \) > \( M_n \), it reflects the presence of longer chains.
3. What is the polydispersity index (\( I \))?
The polydispersity index (\( I \)) is the ratio of the mass average molar mass (\( M_w \)) to the number average molar mass (\( M_n \)): \[ I = \frac{M_w}{M_n} \] It measures the breadth of the molar mass distribution. \( I > 1 \) indicates a broad distribution, with typical values being \( I > 1.5 \) for radical polymerization and \( I \leq 2 \) for step polymerization.
4. How is polymer molar mass determined analytically?
Common methods include: - **Gel Permeation Chromatography (GPC)/Size Exclusion Chromatography (SEC):** Separates polymers by size, providing \( M_n \) and \( M_w \) along with the molar mass distribution. - **Viscometry:** Measures solution viscosity to estimate molar mass, though it is less detailed than GPC.
5. How does molar mass affect polymer properties?
Higher molar mass polymers generally exhibit greater tensile strength, toughness, and elasticity due to increased chain entanglements. Lower molar mass polymers are more flexible and easier to process. Molar mass also influences melt viscosity and thermal properties like glass transition temperature.
6. What is the impact of molar mass distribution on processing?
A narrow molar mass distribution leads to uniform processing behavior and predictable mechanical properties. A broad distribution can improve processability due to shorter chains while maintaining strength from longer chains.
7. What are typical molar mass ranges for common polymer applications?
Examples include: - Plastic bags/bottles: 100,000–250,000 g/mol - Biomedical materials: 3,000,000–6,000,000 g/mol - Automotive textiles: 10,000–50,000 g/mol - High-strength engineering polymers: 100,000–250,000 g/mol.
8. How does molar mass affect polymer viscosity?
Higher molar mass polymers have higher melt viscosities, which can complicate processing (e.g., extrusion, injection molding) but may enhance structural integrity in the final product.
9. What are typical molar masses for common polymer materials?
Examples include: - HDPE: 100,000–250,000 g/mol - Polycarbonate: 50,000–300,000 g/mol - UHMWPE: 2,000,000–6,000,000 g/mol.
10. Are there tools available for molar mass calculations?
Yes, analytical methods like GPC/SEC and viscometry are widely used in industry. Software tools can also assist in interpreting data and calculating \( M_n \), \( M_w \), and polydispersity index.