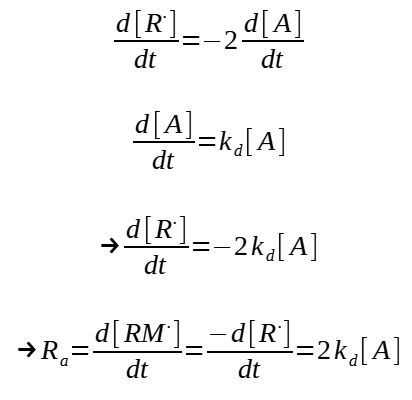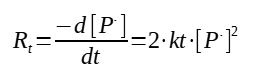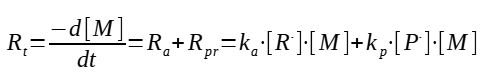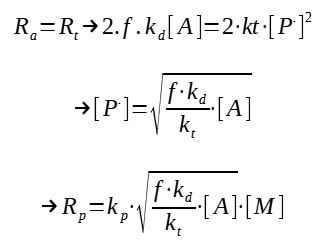Free Radical Polymerization : reaction rate calculation
How to calculate the reaction rate of a polymerization reaction ?
Follow us on Twitter ![]()
Question, remark ? Contact us at contact@myengineeringtools.com
1. Polymerization reaction
2. Initiation rate
3. Propagation rate
4. Termination rate
5. Polymerization rate
Polymerization reactions appear often complex, how to calculate their reaction rate in the case of a free radical polymerization ?
1. Polymerization reaction
In radical polymerization, the following reactions happen :
- Initiation
- Propagation
- Termination
There are also transfer reactions but they are not discussed in this paragraph, therefore now they are considered negligible.
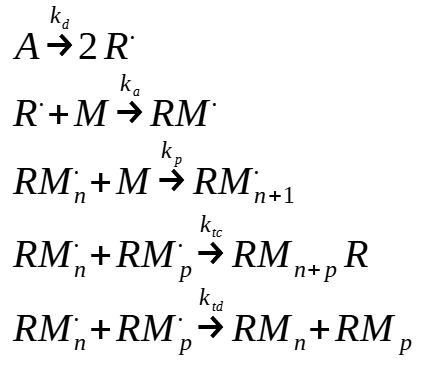
In order to calculate the overall polymerization reaction, it is necessary to express the reaction rate of these different reactions.
2. Initiation rate
The rate of initiation is equal to the rate of production of radical RM
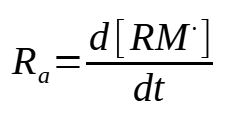
The rate of production of RM. is equal to the rate of consumption of free radical R.
The equations below are given in rate of production.
This expression needs however to be modified to take into consideration that only some of the initiator leads to an actual initiation and then a polymerization reaction. Condering f as being the efficiency of the initiatior (f = 0.3 to 0.8), the rate of initiation is then :
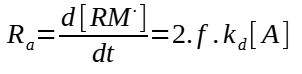
Top 5 Most
Popular
1. Compressor
Power Calculation
2. Pump Power Calculation
3. Pipe Pressure
Drop Calculation
4. Fluid Velocity in pipes
5. Churchill Correlation
(friction factor)
3. Propagation rate
The propagation is actually made of all the successive reactions that allow to add one monomer to the polymer chain.
Considering that the rate constant kp of all the reactions is the same, the propagation rate can be written the following way :
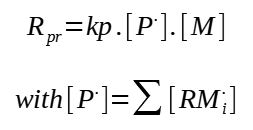
4. Termination rate
The termination reactions are in between 2 radicals, to give "dead" chains, which means that they do not have a radical anymore and thus cannot grow anymore. There are 2 reactions of termination, either 2 radicals give 2 polymer chains (dismutation) or give one polymer chain (recombination), however from a kinetic point of view, the raction rate is expressed the same as 2 radicals react with each other :
5. Polymerization rate
The polymerization rate is defined as the rate of consumption of the monomer :
-d[M] / dt
There are 2 reactions involving the monomer : the initiation and the propagation, and both are consuming the monomer.
If we consider we are producing long chains of polymers, which is normally the case, the expression can be simplified by neglecting the initiation rate, which will be very small compared to all the other propagation reactions.
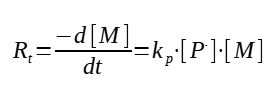
This general expression is however not very practical, indeed, how to determine the concentration in growing chains ?
The following hypothesis can be made : the propagation is very quick, thus the number of radicals is not changing, which means that the initiation rate is equal to the termination rate :
6. Typical Kinetic Constant Values
Values depend strongly on the monomer, initiator, and temperature. For example (at 60 °C):
-
Styrene:
-
-
Methyl methacrylate (MMA):
-
-
Initiator (benzoyl peroxide, BPO):
-
-
Efficiency
-
These values provide a starting point for quick estimates.
7. Worked Example – Styrene with BPO
Let us calculate the polymerization rate at 60 °C:
-
[M] = 5 mol/L
-
[I] = 0.01 mol/L
-
-
-
-
Step 1 – Radical concentration:
[R•]=1×1070.5×1×10−5×0.01Step 2 – Rate of polymerization:
Rp=300×5×7.1×10−8=1.1×10−4mol/L\cdotpsThus, about 0.1 mmol of styrene polymerizes per liter per second under these conditions.
8. Degree of Polymerization and Molecular Weight
The average chain length can be related to the ratio of propagation to termination rates:
Xn=RtRpIn practice, molecular weights are in the range of 104−106g/mol depending on the conditions. Engineers often adjust initiator concentration and temperature to target the desired Mn (number-average molar mass).
9. Practical Considerations for Process Engineers
-
Heat Generation: Radical polymerizations are highly exothermic (~70–100 kJ/mol). Cooling and thermal runaway prevention are critical in reactors.
-
Diffusion Limitations: At high conversion, viscosity increases, and termination becomes diffusion-controlled, leading to autoacceleration (Trommsdorff or gel effect).
-
Industrial Relevance:
-
Bulk polymerization (e.g., PMMA sheets) requires careful heat management.
-
Emulsion polymerization (e.g., latex paints) allows better heat dissipation and higher rates.
-